Chronic obstructive pulmonary disease (COPD) is a condition characterised by progressive airflow obstruction. It is not fully reversible and slow to develop — its severity does not change markedly over short periods — making it less easy to detect. Spirometry is the gold standard for accurate and repeatable measurement of lung function. It is required for COPD to be diagnosed, assessed and monitored correctly.
A spirometer measures the following parameters:
- Forced vital capacity (FVC) — the volume of air that can be exhaled following a single, deep inhalation. It is expressed in litres and as a percentage of the predicted value (based on the patient’s age, height, gender and race)
- Forced expiratory volume in one second (FEV1) — the volume of air exhaled in the first second of a forced exhalation. It is expressed in litres and as a percentage of the predicted value (based on the patient’s age, height, gender and race)
- Relaxed vital capacity (RVC) — the volume of air exhaled during a relaxed blow (litres)
- Forced expiratory ratio — FEV1 expressed as a percentage of FVC (or RVC if that is greater). It is the proportion of an individual’s lung capacity that is exhaled during the first second of a forced blow
Small, hand-held spirometers are available although these only provide digital readings (usually FVC and FEV1 only) — prediction charts and a calculator are then needed to interpret the results.
Portable meters that include an integrated printer are best for use in primary care. These print all readings, calculate predicted values and generate a volume-time graph and a flow-volume graph (which include “expected” curves for the patient). The volume-time graph can be used to monitor whether the patient has performed the test adequately. The shape of the flow-volume curve depends on the mechanical properties of the lung and can give important clues about the diagnosis (see below).
Performing the test
Good quality spirometry relies on the technique of the healthcare professional who performs it. Patients should be asked to sit comfortably and should have the procedure demonstrated to them before starting.
First, the patient must form a tight seal around the disposable mouthpiece with his or her lips. The patient starts with three relaxed blows then performs a series of at least three forced exhalations for as long and as hard as possible — taking a deep breath before each one. A noseclip can be used to prevent air escaping from the nose for the relaxed blows only.
To ensure consistency, the two closest FEV1 readings for the forced blows should differ by no more than 100ml or 5%.
Box 1: Classifying spirometry results
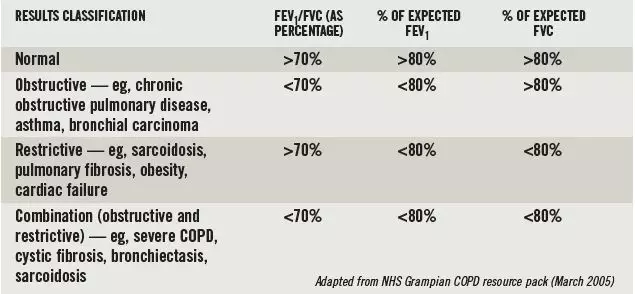
If the patient coughs during the first second of exhaling, or takes a repeat breath during the long exhalation, the test should be repeated. Such occurrences are easily identified by an irregularity on the volume-time curve.
Other problems that can occur include the patient failing to take a deep enough breath before exhaling and the patient not exhaling with sufficient effort. Again, most spirometers identify when this happens and alert the practitioner.
Making a diagnosis
The results of a spirometry test are used to diagnose the cause of airway obstruction as shown in Box 1. It should be noted that spirometry testing alone cannot always differentiate between asthma and COPD. Therefore, a reversibility test must also be carried out. This involves the patient having a baseline spirometry test then being given 4–6 puffs from a salbutamol 100µg inhaler (via a spacer) or 2.5mg of nebulised salbutamol. Fifteen minutes later the spirometry test should be repeated.
The reversibility test can also be carried out using corticosteroids: the patient is given oral prednisolone 30mg, with spirometry repeated after two weeks; or given inhaled beclometasone 1,000µg, with spirometry repeated after six weeks. However, this relies on the patient returning for a second appointment.
If the patient’s FEV1 increases by more than 15% (or 400ml) after the bronchodilator is given, the obstruction is reversible and asthma is more likely. Indeed, patients whose second spirometry results are normal are almost certainly asthmatic. If a 15% improvement is not seen, COPD is a more likely picture.
A full clinical history needs to be taken before the diagnosis can be confirmed. This should include the patient’s past respiratory symptoms (eg, cough, sputum, dyspnoea, wheeze), along with his or her smoking status, occupational history and past medical history. The patient should also be assessed according to the Medical Research Council dyspnoea scale (accessible at www.nice.org.uk).
If the patient’s clinical history identifies any of the following risk factors, a diagnosis of COPD is more likely:
- A history of chronic, progressive wheeze and breathlessness, and a chronic, productive cough
- A history of smoking
- Age over 40 years
- A history of frequent exacerbations of bronchitis
- Breathlessness on exertion
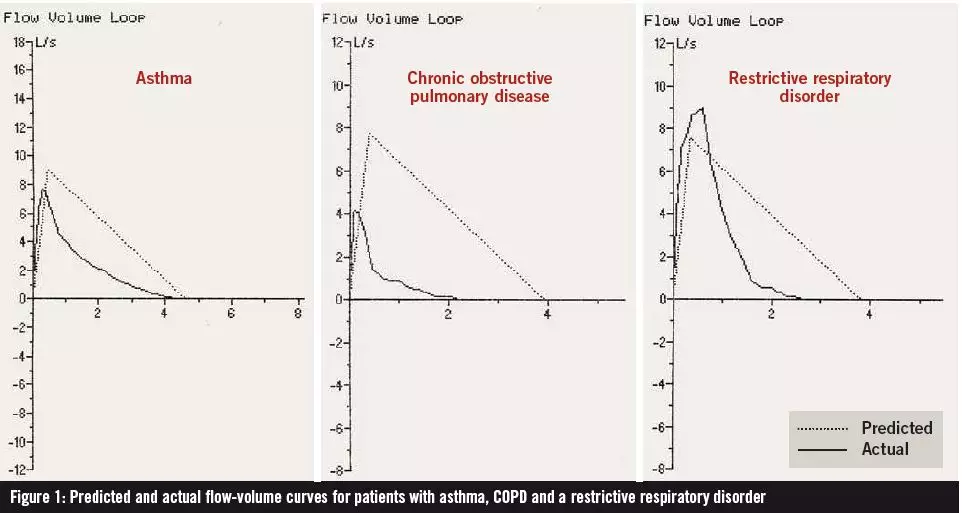
If the spirometer that you use produces a flow-volume graph, this can provide useful information for diagnosis. Figure 1 shows the difference between the flow volume curves for patients with mild asthma, moderate COPD and a restrictive disorder.
Severity
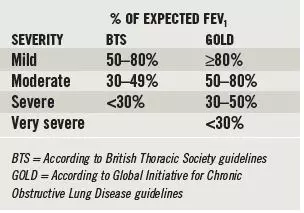
FEV1 — as a percentage of its expected value — is used to classify the severity of obstructive respiratory disease. However, the severity assigned to a patient will differ depending on which clinical guideline is used (see Box 2).
Box 2: Disease severity
Further reading
- National Institute for Health and Clinical Excellence. Chronic obstructive pulmonary disease: national clinical guideline on management of chronic obstructive pulmonary disease in adults in primary and secondary care. Thorax 2004;59(s1):1–232.
- Global Initiative for Chronic Obstructive Lung Disease. Pocket guide to COPD diagnosis, management and prevention. February 2009. www.goldcopd.com (accessed 9 September 2009).
- General Practice Airways Group. GPIAG opinion number 1 (version 2): Spirometry. www.gpiag.org (accessed 9 September 2009).
